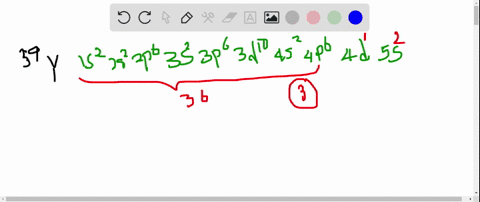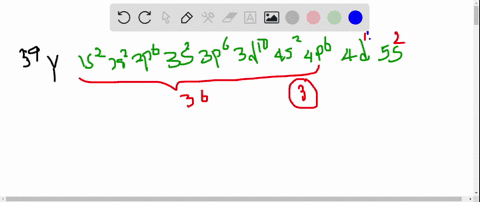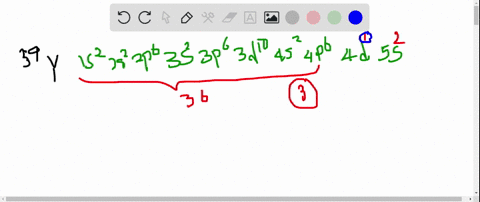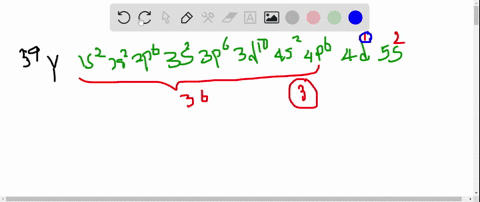
So thats 5 orbitals and. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 4 5s 1.
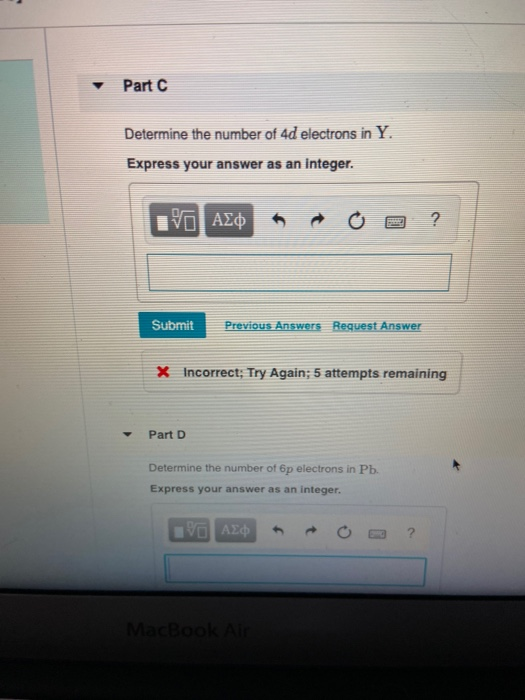
Solved Part C Determine The Number Of 4d Electrons In Y Chegg Com
For the d subshell you have m_l -2 -1 0 1 2 Finally the spin quantum number m_s which denotes the spin of the electron can take two possible values m_s 12 - 12 You now have all the information that you need to write the sets of quantum numbers that can describe an electron located on the 4th energy level in the 4d subshell.
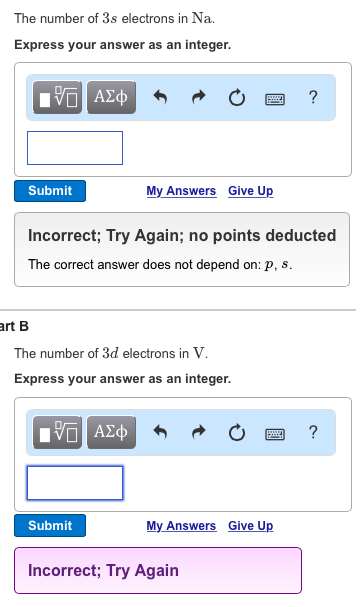
. Name an element in the fourth period row of the periodic table with. 5 attempts remaining Part D Determine the number of 6p electrons in Pb. See answer 1 Best Answer.
Provide an appropriate responseMissouri charges a 6 sales tax and Springfield MO charges an additional 655 city sales tax. Two 3s Electrons and No 3p Electrons. Four 3p Electrons C.
4d 1 5s 2. 1 Give the chemical symbol of an element in the third period row of the periodic table with two 3s electrons and no 3p electrons. Chemistry questions and answers.
11 rows Atomic Number Symbol Name Electron Configuration Filling Orbital Valence Electrons. The number of 4d electrons in Y. Submit Previous Answers Request Answer X Incorrect.
The number of 3d electrons in Cr c. The maximum number of electrons that can occupy and s subshell or sublevel is 2. Each orbital of a subshell can hold 2 electrons.
A 1000 kg rocket without an internal propulsion system is launched with an initial speed of 700 x 102 ms. Determine the number of valence electrons in each element. 4dseries consists of elements from Y atomic number 39 to Cd atomic number 48.
Az D Submit Previour Aneli RequestAnswef Incorrect. Use the periodic table to determine each quantity. The valence electrons for Mo are 5s2 4d4.
The number of 3d electrons in Cr. The electron configuration shows that the last shell of yttrium has two electrons and the d-orbital has a total of an electron. The number of 4d electrons in Y.
10 AED Om. Pant C Determine the number of 4d electrons in Y. So the maximum number of electrons.
The number of 6p electrons in Pb. SoThere are 5 orbitals each consisting of 2 electrons. The 4f can hold up to 14 electrons.
Three Valence Electrons B. IV AL O. 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 1 5s 2.
By signing up youll get thousands of step-by-step solutions to. Use the periodic table to determine each quantity. Express your answer as an integer.
For the transition element the valence electrons have to be determined by adding the total electron of the d-orbital to the electron in the last shell of the atom. The number of 3s electrons in Mg b. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.
1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p. 4d 2 5s 2. The number of 3s electrons in Mg.
Express your answer as an integer. Part C Determine the number of 4d electrons in Y. The concept of electronic configuration has replaced the older concept of valency and valence electrons.
Determine the number of real-number solutions to the equation -2x2 - x - 5 0 given the graph of y -2x2 - x - 5. As d subshell can hold a maximum of 10 electrons. Muxakara and 3 more users found this answer helpful.
How many total electrons can be contained in the 4d. The number of 4d electrons in Y. In level 4 there is 4s 4p 4d and 4f.
1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 2 5s 2. Solution for Use the periodic table to determine quantity. Six 3p Electrons D.
The number of 4d electrons in Y. Use the periodic table to determine the number of 4d electrons in Y. Find step-by-step Chemistry solutions and your answer to the following textbook question.
Because a d subshell corresponds to an l value of 2 m_l can be -2-1012. What is the maximum number of electrons that can occupy the 4th electron shell. Problem 52 Medium Difficulty.
Attempts remaining Part D Determine the number of 6p electrons in Pb Express your answer integer. 4d 4 5s 1. The number of 6p electrons in Pb.
4s2 4p6 4d10 4f14 32 electrons. Determine the maximum height attained by the rocket. Therefore the valence electrons of yttriumY are three.
Express your answer as an Integer. The number of 4d electrons in Y.
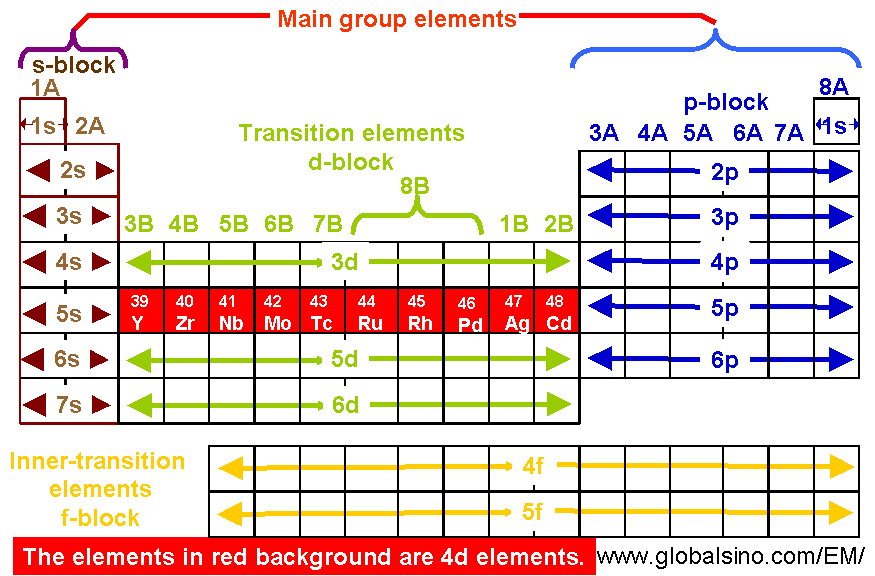
4d Elements In Periodic Table Structure Of The Periodic Table Practical Electron Microscopy And Database An Online Book
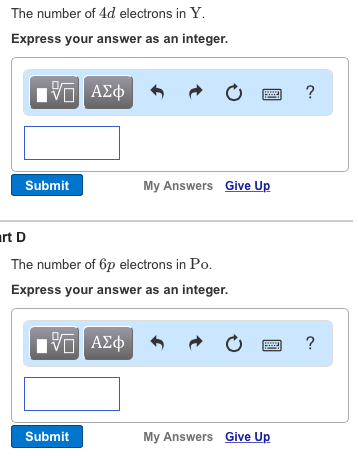
Solved The Number Of 4d Electrons In Y Express Your Answer Chegg Com
Solved How Many 4d Electrons Are Found In Each Of The Following Elements Begin Array Ll Text A Ytrium Z 39 Text C Strontium Z 38 Text B Zirconium Z 40

Solved The Number Of 4d Electrons In Y Express Your Answer Chegg Com
0 Comments